-
EVIDENCE QUALITY
-
-
-
-
-
-
STUDY CONCERNS
-
-
-
-
-
-
ROR
-
-
-
-
-
-
STUDY LIMITATIONS
-
-
-
-
-
-
DYDROGESTERONE IN CONTEXT
-
-
-
-
-
-
INFORMED CARE
-
-
-
-
-
-
KEY TAKEAWAYS
-
-
-

Evaluating Dydrogesterone Safety Evidence:
A Review of Henry et al. (2025)
References
- Cooper, I.D. (2016). “Evidence ladder and the journal of the medical library association,” Journal of the Medical Library Association, 104(4), p. 262.
- Parazzini, F., Cantarutti, A. and Esposito, G. (2025). “Unmasking the risk: clinical trials versus real-world evidence on dydrogesterone and birth defects,” Human Reproduction Open, 2025(1).
- Henry, A. et al. (2025). “Birth defects reporting and the use of dydrogesterone: a disproportionality analysis from the World Health Organization pharmacovigilance database (VigiBase),” Human Reproduction Open, 2025(1).
- Uppsala Monitoring Centre (2021). “Guideline for using VigiBase data in studies,” Version 4, March 15, 2021.
- Henry, A. et al. (2023). “0-150 Birth defects reporting and the use of oral dydrogesterone in assisted reproductive technology: a global pharmacovigilance study,” Human Reproduction, 38(Supplement_1).
- Luke, B. et al. (2021). “The risk of birth defects with conception by ART,” Human Reproduction (Oxford, England), 36(1), pp. 116–129.
- Pethő, B. et al. (2023). “Maternal age is highly associated with non-chromosomal congenital anomalies: Analysis of a population-based case-control database,” BJOG: An International Journal of Obstetrics and Gynaecology, 130(10), pp. 1217–1225.
- Khoury, M.J. and Erickson, J.D. (1993). “Recurrent pregnancy loss as an indicator for increased risk of birth defects: a population-based case-control study,” Paediatric and Perinatal Epidemiology, 7(4), pp. 404–416.
References
- Baker, V.L. et al. (2010). “Multivariate analysis of factors affecting probability of pregnancy and live birth with in vitro fertilization: An analysis of the Society for Assisted Reproductive Technology Clinic Outcomes Reporting System,” Fertility and Sterility, 94(4), pp. 1410–1416.
- Podzolkova, N. et al. (2016). “Dydrogesterone treatment for menstrual-cycle regularization in routine clinical practice: A multicenter observational study,” Gynecological Endocrinology, 32(3), pp. 246–249.
- 29th Periodic Safety Update Report: Dydrogesterone (22 APR 2021 – 21 APR 2024).
- Griesinger, G. et al. (2020). “Dydrogesterone as an oral alternative to vaginal progesterone for IVF luteal phase support: A systematic review and individual participant data meta-analysis,” PLOS ONE, 15(11), e0241044.
- Katalinic, A. et al. (2022). “A critical appraisal of safety data on dydrogesterone for the support of early pregnancy: a scoping review and meta-analysis,” Reproductive BioMedicine Online, 45(2), pp. 365–373.
- Katalinic, A. et al. (2024). “No additional risk of congenital anomalies after first-trimester dydrogesterone use: a systematic review and meta-analysis,” Human Reproduction Open, 2024(1).
- Chan, D.M.K. et al. (2016). “A randomized double-blind controlled trial of the use of dydrogesterone in women with threatened miscarriage in the first trimester: study protocol for a randomized controlled trial,” Trials, 17(1), p. 408.
References
- Tournaye, H. et al. (2017). “A Phase III randomized controlled trial comparing the efficacy, safety and tolerability of oral dydrogesterone versus micronized vaginal progesterone for luteal support in in vitro fertilization,” Human Reproduction, 32(5), pp. 1019–1027.
- Griesinger, G., Blockeel, C., Sukhikh, G.T., et al. (2018). “Oral dydrogesterone versus intravaginal micronized progesterone gel for luteal phase support in IVF: a randomized clinical trial,” Human Reproduction, 33(12), pp. 2212–2221.
- Yang, D.Z. et al. (2020). “A Phase III randomized controlled trial of oral dydrogesterone versus intravaginal progesterone gel for luteal phase support in in vitro fertilization (Lotus II): results from the Chinese mainland subpopulation,” Gynecological Endocrinology, 36(2), pp. 175–183.
- Li, L. et al. (2024). “The maternal drug exposure birth cohort (DEBC) in China,” Nature Communications, 15(1), pp. 1–14.
- Zaquout, M. et al. (2015). “The Impact of Oral Intake of Dydrogesterone on Fetal Heart Development During Early Pregnancy,” Pediatric Cardiology, 36(7), pp. 1483–1488.
Scroll down to start, or click an icon to jump directly to a section
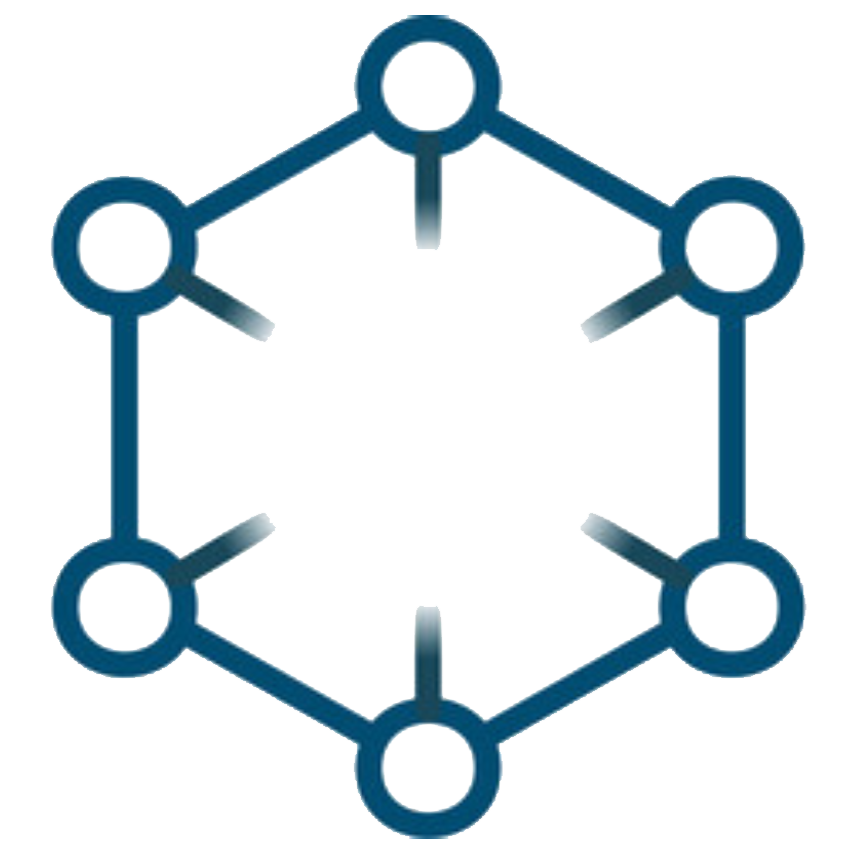
CLINICAL DECISIONS ARE BUILT ON
LAYERS OF
EVIDENCE
Not All
EVIDENCE
IS CREATED EQUAL
IN SCIENTIFIC PRACTICE,
THE QUALITY AND TYPE OF EVIDENCE ARE AS CRITICAL AS THE DATA ITSELF
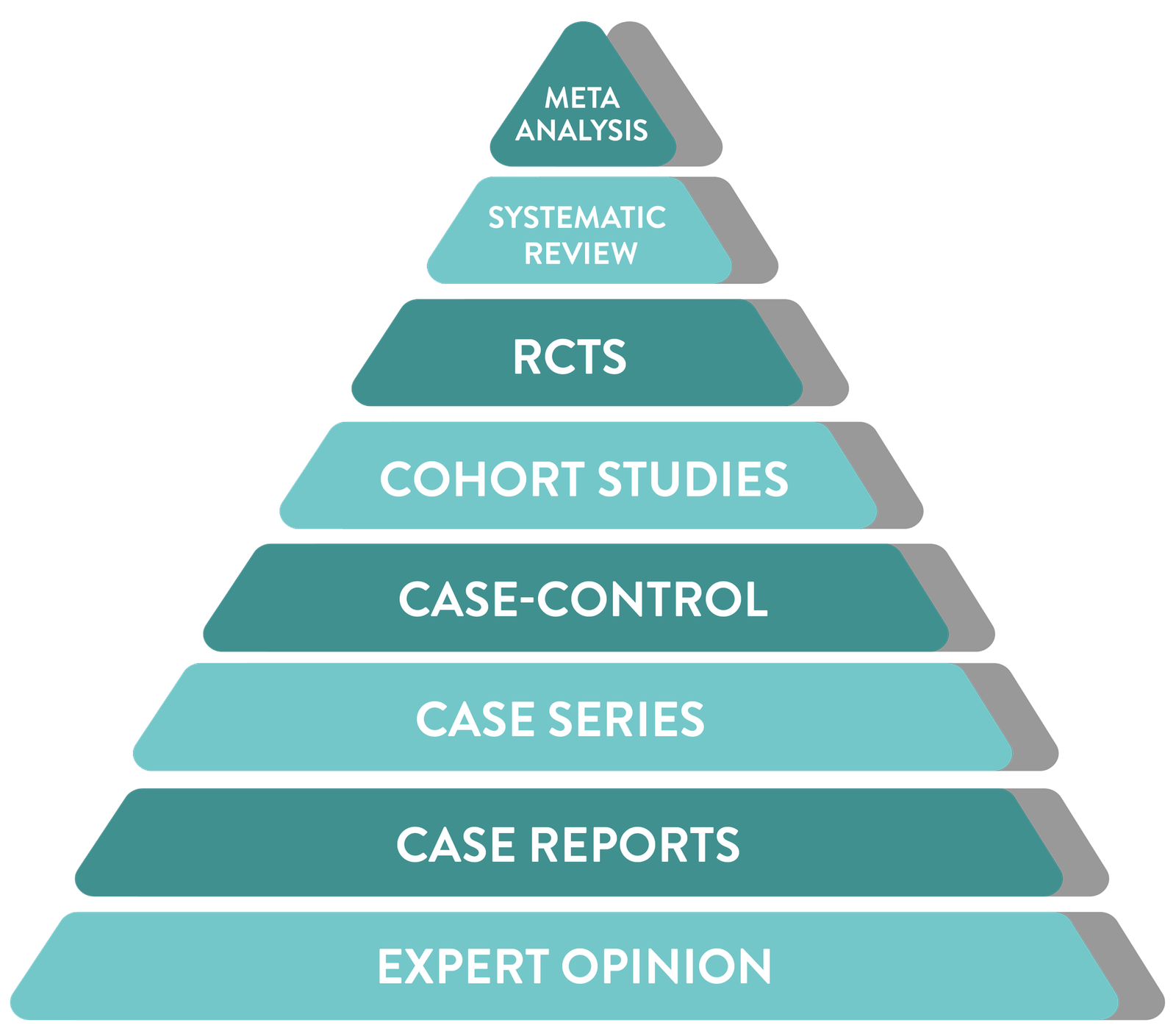
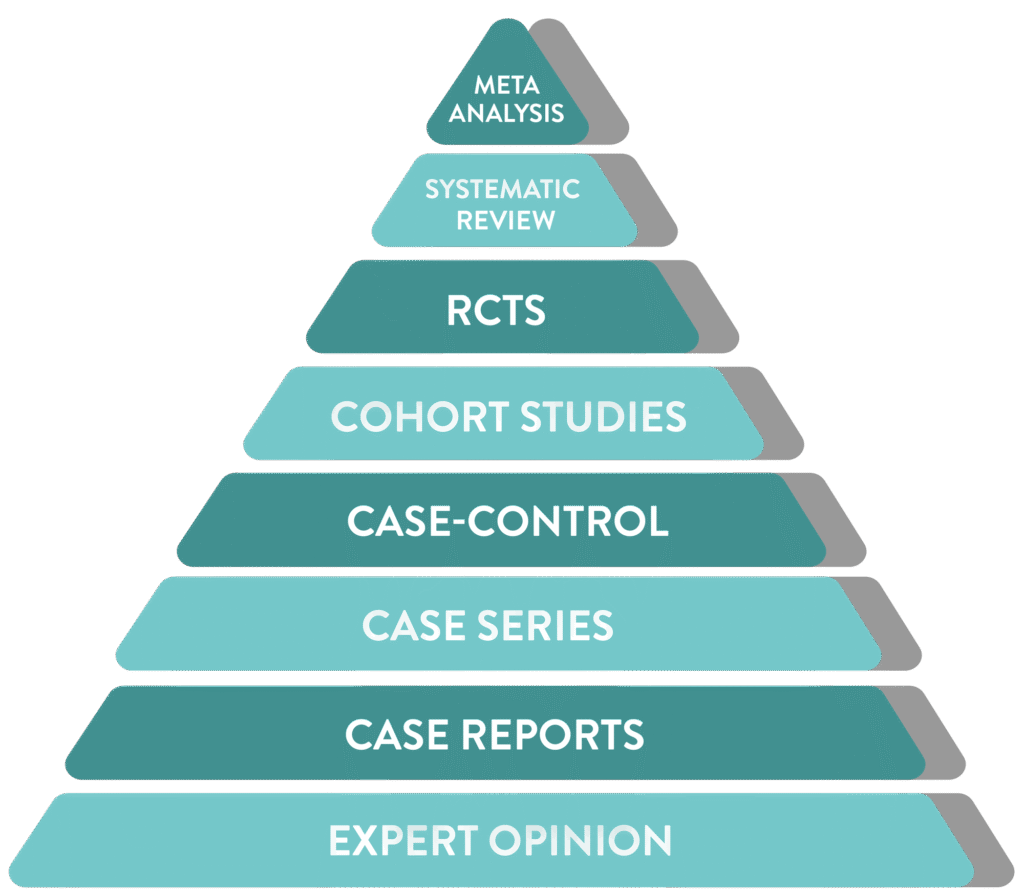
From individual case reports to large-scale meta-analyses, each successive level of study design increases confidence, robustness, and reliability

Incorporation of all available data with non-critical bias
Meta
Analysis
Analysis
Statistically combines data from multiple studies
Systematic
Review
Review
Summarizes all relevant studies on a topic
RCTs
Randomized trial comparing interventions, often to a control or placebo group
Cohort Studies
Follows groups over time to assess outcomes
Case-Control
Compares patients with a condition to those without
Case Series
Summary of similar cases in multiple patients
Case Reports
Detailed report on a single patient
Expert Opinion
Insights based on clinical experience rather than research

Henry et al. 2025  analysis of data until 20213
analysis of data until 20213
Hover your mouse over the pyramid to explore the hierarchy of scientific evidence.

where does a
pharmacovigilance signal fit in?